Advances in boron compounds: Author's perspectives on their role in biotechnology from antimicrobial agents to cancer therapy
DOI:
https://doi.org/10.62063/rev-203143Keywords:
enzyme inhibition, antimicrobial effects, boron compounds, biotechnological applications, boron neutron capture therapyAbstract
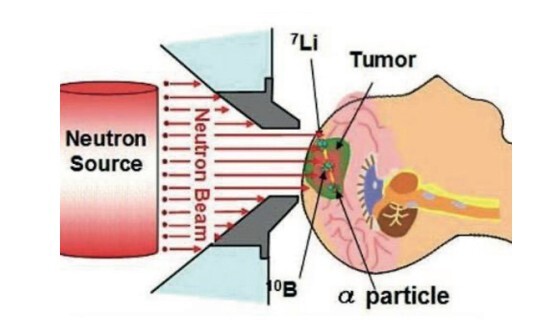
Boron compounds, both organic and inorganic, have emerged as versatile and promising materials with wide-ranging applications in medicinal chemistry, catalysis, and materials science. Organic boron compounds, including heterocyclic aminoboron derivatives and boronic acids, have shown significant potential as antimicrobial and anticancer agents, with research highlighting their effectiveness in treating infections and inhibiting cancer cell proliferation. The ongoing research, including the author's own studies, demonstrates significant promise regarding inorganic boron compounds, which possess a considerable potential that cannot be overlooked. Boron Neutron Capture Therapy (BNCT) has garnered attention for its targeted approach in cancer therapy, facilitated by the development of innovative boron-based drug delivery systems. Inorganic boron compounds, on the other hand, have contributed to advancements in catalytic processes, material stability, and electronic properties, offering opportunities for applications in organic electronics, flame-retardant materials, and drug development. The unique chemical reactivity of boron compounds, including their ability to inhibit enzymes such as β-lactamases and histone deacetylases, positions them as valuable tools in combating antibiotic resistance and cancer. This review provides a comprehensive overview of the properties, applications, and therapeutic potential of boron compounds, emphasizing their role in drug delivery, enzyme inhibition, and antimicrobial development. Ongoing research into the structural modification and functionalization of boron-based compounds continues to expand their scope, positioning them as key candidates for the development of novel therapeutic agents in biotechnology and medicine.
References
Adamczyk-Woźniak, A., Borys, K. M., & Sporzyński, A. (2016). Recent developments in the chemistry and biological applications of boronic acids. Chemical Reviews, 116(9), 5083-5131.
Adams, J. (2004). The proteasome: a suitable antineoplastic target. Nature Reviews Cancer, 4(5), 349-360. https://doi.org/10.1038/nrc1361
Ahrens, V., Frank, R., Boehnke, S., Schütz, C., Hampel, G., Iffland, D., Bings, N.H., Hey-Hawkins, E.H., & Beck-Sickinger, A. (2014). Receptor-mediated uptake of boron-rich neuropeptide y analogues for boron neutron capture therapy. Chemmedchem, 10(1), 164-172. https://doi.org/10.1002/cmdc.201402368
Akbari, N., Ostadrahimi, A., Tutunchi, H., Pourmoradian, S., Farrin, N., Najafipour, F., Soleimanzadeh, H., Kafil, B., & Mobasseri, M. (2022). Possible therapeutic effects of boron citrate and oleoylethanolamide supplementation in patients with covid-19: a pilot randomized, double blind, clinical trial. Journal of Trace Elements in Medicine and Biology, 71, 126945. https://doi.org/10.1016/j.jtemb.2022.126945
Ali, F., Hosmane, N., & Zhu, Y. (2020). Boron chemistry for medical applications. Molecules, 25(4), 828. https://doi.org/10.3390/molecules25040828
Ando, N., Yamada, T., Narita, H., Oehlmann, N., Wagner, M., & Yamaguchi, S. (2021). Boron-doped polycyclic π-electron systems with an antiaromatic borole substructure that forms photoresponsive B–P Lewis adducts. Journal of the American Chemical Society, 143(26), 9944-9951. https://doi.org/10.1021/jacs.1c04251
Anufriev, S., Sivaev, I., & Nakamura, H. (2020). Two possible ways to combine boron and gadolinium for Gd-guided BNCT: A concept. Phosphorus Sulfur and Silicon and the Related Elements, 195(11), 910-917. https://doi.org/10.1080/10426507.2020.1804151
Ailuno, G., Balboni, A., Caviglioli, G., Lai, F., Barbieri, F., Dellacasagrande, I., Florio, T., & Baldassari, S. (2022). Boron vehiculating nanosystems for neutron capture therapy in cancer treatment. Cells, 11(24), 4029. https://doi.org/10.3390/cells11244029
Barth, R., Mi, P., & Yang, W. (2018a). Boron delivery agents for neutron capture therapy of cancer. Cancer Communications, 38(1), 1-15. https://doi.org/10.1186/s40880-018-0299-7
Barth, R., Zhang, Z., & Liu, T. (2018b). A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Communications, 38(1), 1-7. https://doi.org/10.1186/s40880018-0280-5
Brem, J., Cain, R., Cahill, S., McDonough, M., Clifton, I., Jiménez-Castellanos, J., Avison, M.B., Spencer, J., Fishwick, C.W.G., & Schofield, C. (2016). Structural basis of metallo-β-lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nature Communications, 7(1). https://doi.org/10.1038/ncomms12406
Cahill, S., Cain, R., Wang, D., Lohans, C., Wareham, D., Oswin, H., Mohammed, J., Spencer, J., Fishwick, C.W.G., McDonough, M.A., Schofield, C.J., & Brem, J. (2017). Cyclic boronates inhibit all classes of β-lactamases. Antimicrobial Agents and Chemotherapy, 61(4). https://doi.org/10.1128/aac.02260-16
Campbell-Verduyn, L., Bowes, E., Li, H., Vallée, A., Vogels, C., Decken, A., Gray, C.A., & Westcott, S. (2014). Heterocyclic aminoboron compounds as antituberculosis agents. Heteroatom Chemistry, 25(2), 100-106. https://doi.org/10.1002/hc.21141
Das, B. C., Nandwana, N. K., Das, S., Nandwana, V., Shareef, M. A., Das, Y., Saito, M., Weiss, L. M., Almaguel, F., Hosmane, N. S., & Evans, T. (2022). Boron chemicals in drug discovery and development: synthesis and medicinal perspective. Molecules, 27(9), 2615. https://doi.org/10.3390/molecules27092615
Dash, B., Satapathy, R., Bode, B., Reidl, C., Sawicki, J., Mason, A., Maguire, J.A., & Hosmane, N. (2012). “Click” chemistry-mediated phenylene-cored carborane dendrimers. Organometallics, 31(7), 2931-2935. https://doi.org/10.1021/om201255b
Fontaine, F., Héquet, A., Voisin-Chiret, A., Bouillon, A., Lesnard, A., Cresteil, T., Jolivalt, C., & Rault, S. (2014). First identification of boronic species as novel potential inhibitors of the Staphylococcus aureus Nora efflux pump. Journal of Medicinal Chemistry, 57(6), 2536-2548. https://doi.org/10.1021/jm401808n
Frase, H., & Lee, I. (2007). Peptidyl boronates inhibit Salmonella enterica serovar Typhimurium Lon protease by a competitive ATP-dependent mechanism. Biochemistry, 46(22), 6647-6657. https://doi.org/10.1021/bi7002789
Ganbar, G. (2019). Functionally substituted chemical organic compounds: Potential antimicrobial substances. Open Access Journal of Microbiology & Biotechnology, 4(1). https://doi.org/10.23880/oajmb-16000136
Glynn, S., Gaffney, K., Sainz, M., Louie, S., & Petasis, N. (2015). Molecular characterization of the boron adducts of the proteasome inhibitor bortezomib with epigallocatechin-3-gallate and related polyphenols. Organic & Biomolecular Chemistry, 13(13), 3887-3899. https://doi.org/10.1039/c4ob02512a
Hall, D.G. (2011). Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, Second Edition. Edited by Wiley-VCH Verlag GmbH & Co. KGaA.
Hattori, Y., Ishimura, M., Ohta, Y., Takenaka, H., Kawabata, S., & Kirihata, M. (2021). Dodecaborate conjugates targeting tumor cell overexpressing translocator protein for boron neutron capture therapy. Acs Medicinal Chemistry Letters, 13(1), 50-54. https://doi.org/10.1021/acsmedchemlett.1c00377
Heber, E., Hawthorne, M., Kueffer, P., Garabalino, M., Thorp, S., Pozzi, E., Hughes, A.M., Maitz, C.A., Jalisatgi, S.S., Nigg, D.W., Curotto, P., Trivillin, V.A., & Schwint, A. (2014). Therapeutic efficacy of boron neutron capture therapy mediated by boron-rich liposomes for oral cancer in the
hamster cheek pouch model. Proceedings of the National Academy of Sciences, 111(45), 16077-16081. https://doi.org/10.1073/pnas.1410865111
Heber, E., Kueffer, P., Lee, M., Hawthorne, M., Garabalino, M., Molinari, A., Nigg, D.W., Bauer, W., Hughes, A.M., Pozzi, E.C.C., Trivillin, V.A., & Schwint, A. (2012). Boron delivery with liposomes for boron neutron capture therapy (BNCT): Biodistribution studies in an experimental model of
oral cancer demonstrating therapeutic potential. Radiation and Environmental Biophysics, 51(2), 195-204. https://doi.org/10.1007/s00411-011-0399-0
Heide, F., McDougall, M., Harder-Viddal, C., Roshko, R., Davidson, D., Wu, J., Aprosoff, C., Moya Torres, A., Lin, F.,& Stetefeld, J. (2021). Boron rich nanotube drug carrier system is suited for boron neutron capture therapy. Scientific Reports, 11(1). https://doi.org/10.1038/s41598-021-95044-0
Issa, F., Kassiou, M., & Rendina, L. (2011). Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chemical Reviews, 111(9), 5701-5722. https://doi.org/10.1021/cr2000866
Kaniowski, D., Suwara, J., Ebenryter-Olbińska, K., Jakóbik-Kolon, A., & Nawrot, B. (2022). EGFR targeted cellular delivery of therapeutic nucleic acids mediated by boron clusters. International Journal of Molecular Sciences, 23(23), 14793. https://doi.org/10.3390/ijms232314793
Koganei, H., Ueno, M., Tachikawa, S., Tasaki, L., Ban, H., Suzuki, M., Shiraishi, K., Kawano, K., Yokoyama, M., Maitani, Y., Ono, K., & Nakamura, H. (2012). Development of high boron content liposomes and their promising antitumor effect for neutron capture therapy of cancers. Bioconjugate Chemistry, 24(1), 124-132. https://doi.org/10.1021/bc300527n
Koldemir-Gündüz, M., Aydın, H., Berikten, D., Kaymak, G., Köse, D., & Arslantaş, A. (2021). Synthesis of new boron derived compounds; anticancer, antioxidant and antimicrobial effect in vitro glioblastoma tumor model. Journal of Korean Neurosurgical Society, 64(6), 864-872. https://doi.org/10.3340/jkns.2021.0032
Kollár, L. (2024). Boronic acid inhibitors of penicillin-binding protein 1b: Serine and lysine labelling agents. Journal of Enzyme Inhibition and Medicinal Chemistry, 39(1). https://doi.org/10.1080/14756366.2024.2305833
Krajnc, A., Brem, J., Hinchliffe, P., Calvopiña, K., Panduwawala, T., Lang, P., Kamps, J.J.A.G., Tyrrell, J.M., Widlake, E., Saward, B.G., Walsh, T.R., Spencer, J., & Schofield, C. (2019). Bicyclic boronate VNRX-5133 inhibits metallo- and serine-β-lactamases. Journal of Medicinal Chemistry, 62(18), 8544-8556. https://doi.org/10.1021/acs.jmedchem.9b00911
Li, X., Wang, X., Zhang, J., Hanagata, N., Wang, X., Weng, Q., Ito, A., Bando, Y., & Golberg, D. (2017). Hollow boron nitride nanospheres as boron reservoir for prostate cancer treatment. Nature Communications, 8(1). https://doi.org/10.1038/ncomms13936
Luderer, M., Muz, B., Puente, P., Chavalmane, S., Kapoor, V., Marcelo, R., Biswas, P., Thotala, D., Rogers, B., & Azab, A. (2016). A hypoxia-targeted boron neutron capture therapy agent for the treatment of glioma. Pharmaceutical Research, 33(10), 2530-2539. https://doi.org/10.1007/s11095-016-1977-2
Milani, P., Demasi, M., Rezende, L., Amaral, A., & Andrade, L. (2014). Synthesis of L-cysteine-based boron compounds and their evaluation as proteasome inhibitors. New Journal of Chemistry, 38(10), 4859-4871. https://doi.org/10.1039/c4nj00612g
Mergheş, P., Ilia, G., Hulka, I., Chiriac, V., Varan, N., & Simulescu, V. (2022). The influence of boron on the structure and properties of hybrid compounds containing zirconium and phosphorus. Gels, 8(10), 667. https://doi.org/10.3390/gels8100667
El-Zaria, M., & Nakamura, H. (2009). New strategy for synthesis of mercaptoundecahydrododecaborate derivatives via click chemistry: possible boron carriers and visualization in cells for neutron capture therapy. Inorganic Chemistry, 48(24), 11896-11902. https://doi.org/10.1021/ic902033c
Nakase, I., Aoki, A., Sakai, Y., Hirase, S., Ishimura, M., Takatani-Nakase, T., Hattori, Y., & Kirihata, M. (2020). Antibody-based receptor targeting using an FC-binding peptide-dodecaborate conjugate and macropinocytosis induction for boron neutron capture therapy. Acs Omega, 5(36), 22731-22738. https://doi.org/10.1021/acsomega.0c01377
Saraç, N., Uğur, A., Boran, R., & Elgin, E. (2015). The use of boron compounds for stabilization of lipase from Pseudomonas aeruginosa ES3 for the detergent industry. Journal of Surfactants and Detergents, 18(2), 275-285. https://doi.org/10.1007/s11743-014-1624-3
Scorei, I. and Popa, R. (2010). Boron-containing compounds as preventive and chemotherapeutic agents for cancer. Anti-Cancer Agents in Medicinal Chemistry, 10(4), 346-351. https://doi.org/10.2174/187152010791162289
Seneviratne, D., Saifi, O., Mackeyev, Y., Malouff, T., & Krishnan, S. (2023). Next-generation boron drugs and rational translational studies driving the revival of bnct. Cells, 12(10), 1398. https://doi.org/10.3390/cells12101398
Suzuki, N., Suzuki, T., Ota, Y., Nakano, T., Kurihara, M., Okuda, H., Yamori, T., Tsumoto, H., Nakagawa, H., & Miyata, N. (2009). Design, synthesis, and biological activity of boronic acid based histone deacetylase inhibitors. Journal of Medicinal Chemistry, 52(9), 2909-2922. https://doi.org/10.1021/jm900125m
Tamanoi, F., Chinnathambi, S., Laird, M., Komatsu, A., Birault, A., Takata, T., Doan, T. L.-H., Mai, N. X. D., Raitano, A., Morrison, K., Suzuki, M., & Matsumoto, K. (2021). Construction of boronophenylalanine-loaded biodegradable periodic mesoporous organosilica nanoparticles for bnct cancer therapy. International Journal of Molecular Sciences, 22(5), 2251. https://doi.org/10.3390/ijms22052251
Thamilselvan, G., Sarveswari, H., Vasudevan, S., Stanley, A., Shanmugam, K., Vairaprakash, P., & Solomon, A.P. (2021). Development of an antibiotic resistance breaker to resensitize drug resistant staphylococcus aureus: in silico and in vitro approach. Frontiers in Cellular and Infection Microbiology, 11. https://doi.org/10.3389/fcimb.2021.700198
Türkez, H., Geyikoğlu, F., Tatar, A., Keleş, M., & Özkan, A. (2007). Effects of some boron compounds on peripheral human blood. Zeitschrift Für Naturforschung C, 62(11-12), 889-896. https://doi.org/10.1515/znc-2007-11-1218
Unlu, S., Doğan, Ş., & Doğan, M. (2014). Comparative study of boron compounds and aluminum trihydroxide as flame retardant additives in epoxy resin. Polymers for Advanced Technologies, 25(8), 769-776. https://doi.org/10.1002/pat.3274
Vedelago, J., Mattea, F., Triviño, S., Montesinos, M., Keil, W., Valente, M., & Romero, M. (2021). Smart material based on boron crosslinked polymers with potential applications in cancer radiation therapy. Scientific Reports, 11(1). https://doi.org/10.1038/s41598-021-91413-x
Wang, S., Blaha, C., Santos, R., Huynh, T., Hayes, T., Beckford-Vera, D., Blecha, J.E., Hong, A.S., Miko Fogarty, Hope, T.A., Raleigh, D.R., Wilson, D.M., Evans, M.J., VanBrocklin, H.F., Ozawa, T., & Flavell, R. (2019). Synthesis and initial biological evaluation of boron-containing prostate-specific membrane antigen ligands for treatment of prostate cancer using boron neutron capture therapy. Molecular Pharmaceutics, 16(9), 3831-3841. https://doi.org/10.1021/acs.molpharmaceut.9b00464
Wang, Y. and Spokoyny, A. (2022). Abiotic main group pharmacophore renders a new class of antimicrobial agents. Acs Central Science, 8(3), 309-311. https://doi.org/10.1021/acscentsci.2c00187
Worm, D., Els-Heindl, S., Kellert, M., Kuhnert, R., Saretz, S., Koebberling, J., Riedl, B., Hey-Hawkins, E., & Beck-Sickinger, A. (2018). A stable meta-carborane enables the generation of boron-rich peptide agonists targeting the ghrelin receptor. Journal of Peptide Science, 24(10). https://doi.org/10.1002/psc.3119
Worm, D., Hoppenz, P., Els-Heindl, S., Kellert, M., Kuhnert, R., Saretz, S., Köbberling, J., Riedl, B., Hey-Hawkins, E., & Beck-Sickinger, A. (2019). Selective neuropeptide y conjugates with maximized carborane loading as promising boron delivery agents for boron neutron capture therapy. Journal of Medicinal Chemistry, 63(5), 2358-2371. https://doi.org/10.1021/acs.jmedchem.9b01136
Xiong, H., Wang, X., Zhou, D., Qi, Y., Xie, Z., Chen, X., Jing, X., & Huang, Y. (2016). Amphiphilic polycarbonates from carborane-installed cyclic carbonates as potential agents for boron neutron capture therapy. Bioconjugate Chemistry, 27(9), 2214-2223. https://doi.org/10.1021/acs.bioconjchem.6b00454
Yang, W., Gao, X., & Wang, B. (2003). Boronic acid compounds as potential pharmaceutical agents. Medicinal Research Reviews, 23(3), 346-368. https://doi.org/10.1002/med.10043
Yuan, Z., Zhang, J., Guo, J., Xu, Y., Duan, K., Zheng, J., Wan, H., Yuan, Z., & Chen, H. (2019). Application of nitroimidazole–carbobane-modified phenylalanine derivatives as dual-target boron carriers in boron neutron capture therapy. Molecular Pharmaceutics, 17(1), 202-211. https://doi.org/10.1021/acs.molpharmaceut.9b00898
Zhi, S., Wang, Y., Li, Y., Cheng, J., & Yang, G. (2018). Linking 1d transition-metal coordination polymers and different inorganic boron oxides to construct a series of 3d inorganic–organic hybrid borates. Inorganic Chemistry, 57(3), 1350-1355. https://doi.org/10.1021/acs.inorgchem.7b02765
Zhu, D., Hunter, C., Baird, S., Davis, B., Bos, A., Geier, S., Vogels, C.M., Decken, A., Gray, C.A., & Westcott, S. (2017). Synthesis and antimicrobial properties of cyclic fluorodiamines containing boronate esters. Heteroatom Chemistry, 28(6). https://doi.org/10.1002/hc.21405
Zu, B., Guo, Y., & He, C. (2021). Catalytic enantioselective construction of chiroptical boron stereogenic compounds. Journal of the American Chemical Society, 143(39), 16302-16310.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Gulsah Celik Gul

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







