Current issue
Online first
About
About the Journal
Aims & Scope
Editorial office
Abstracting and indexing
Publisher
Contact
For Authors
Author guideline
Manuscript template
Policies
Peer review policy
Ethical principles and publication policy
Language policy
Conflict of interest statement
Copyright statement
Open access policy
Archiving policy
Privacy statement
Referee and editor's guide
Price policy
License
Archive
BIOCHEMISTRY, GENETICS & MOLECULAR BIOLOGY / REVIEW
Hippocampal contributions to biologic, behavioral and cognitive deficits in autism: An update review
1
Electro neurophysiology, Vocational School of Health Sciences, Üsküdar University, Istanbul, Türkiye
2
Department of Physiology, Faculty of Medicine, Üsküdar University, Istanbul, Türkiye
3
Medical Laboratory Techniques, Vocational School of Health Sciences, Üsküdar University, Istanbul, Türkiye
4
Department of Molecular Biology and Genetics, Üsküdar University, Istanbul, Türkiye
Submission date: 2024-07-30
Final revision date: 2024-10-01
Acceptance date: 2024-10-03
Online publication date: 2024-10-15
Publication date: 2025-01-25
Corresponding author
Mesut Karahan
Medical Laboratory Techniques, Vocational School of Health Sciences, Üsküdar University, Istanbul, Türkiye
Medical Laboratory Techniques, Vocational School of Health Sciences, Üsküdar University, Istanbul, Türkiye
Euchembioj Rev. 1(1), Article e25006 (2025)
KEYWORDS
TOPICS
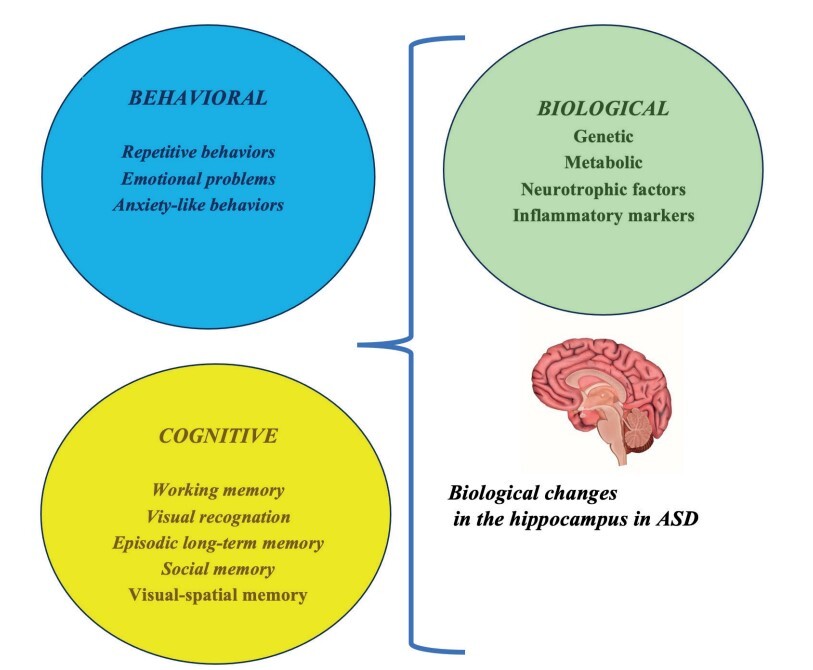
ABSTRACT
The most characteristic symptoms for the diagnosis of autism spectrum disorder (ASD) and the future life of the individual are deterioration in
social communication and stereotyped or repetitive behaviors. ASD is associated with diverse atypical difficulties, including memory, learn
ing, language, emotion, and cognitive impairment. Consequently, the hippocampus is important for memory, learning, language ability, emo
tional regulation, and cognitive mapping. Thus, the hippocampus plays an influential role in the pathophysiological mechanisms of ASD. Here,
we provide an updated review of hippocampal structural and functional abnormalities and highlight the hippocampus as an important area for
future research.
ACKNOWLEDGEMENTS
None.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
PEER REVIEW INFORMATION
Article has been screened for originality
Externally peer reviewed.
REFERENCES (59)
1.
Ajram, L.A., Pereira, A.C., Durieux, A.M.S., Velthius, H.E., Petrinovic, M.M., & McAlonan, G.M. (2019). The contribution of [1H] magnetic resonance spectroscopy to the study of excitationinhibition in autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 89, 236-244. https://doi.org/10.1016/j.pnpb....
2.
Allsop, S.A., Wichmann, R., Mills, F., Burgos-Robles, A., Chang, C.J., Felix-Ortiz, A.C., Vienne, A., Beyeler, A., Izadmehr, E.M., Glober, G., Cum, M.I., Stergiadou, J., Anandalingam, K.K., Farris, K., Namburi, P., Leppla, C.A., Weddington, J.C., Nieh, E.H., Smith, A.C., Ba, D., Brown, E.N., Tye, K.M. (2018). Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell, 173(6), 1329-1342.e18. https://doi.org/10.1016/j.cell....
3.
Bangerter, A., Ness, S., Aman, M.G., Esbensen, A.J., Goodwin, M.S., Dawson, G., Hendren, R., Leventhal, B., Khan, A., Opler, M., Harris, A., & Pandina, G. (2017). Autism Behavior Inventory: A Novel Tool for Assessing the Core and Associated Symptoms of Autism Spectrum Disorder. Journal of Child and Adolescent Psychopharmacology, 27(9), 814-822. https://doi.org/10.1089/cap.20....
4.
Barbosa, A.G., Pratesi, R., Paz, G.S.C., Dos Santos, M.A.A.L., Uenishi, R.H., Nakano, E.Y., Gandolfi, L., & Pratesi, C.B. (2020). Assessment of serum BDNF levels as a diagnostic marker in children with autism spectrum disorder. Scientific Reports,10, 17348. https://doi.org/10.1038/s41598....
5.
Barón-Mendoza, I., Mejía-Hernández, M., Hernández-Mercado, K., Guzmán-Condado, J., Zepeda, A., & González-Arenas, A. (2024). Altered hippocampal neurogenesis in a mouse model of autismrevealed by genetic polymorphisms and atypical development of newborn neurons. Scientific Reports, 14, 4608. https://doi.org/10.1038/s41598....
6.
Bathina, S., & Das, U.N. (2015). Brain-derived neurotrophic factor and its clinical implications. Archives of Medical Science, 11(6), 1164-78. https://doi.org/10.5114/aoms.2....
7.
Bertoldi, M.L., Zalosnik, M.I., Fabio, M.C., Aja, S., Roth, G.A., Ronnett, G.V., & Degano, A.L. (2019). MeCP2 Deficiency Disrupts Kainate-Induced Presynaptic Plasticity in the mosaic fiber projections in the Hippocampus. Frontiers in Cellular Neuroscience, 13, 286. https://doi.org/10.3389/fncel.....
8.
Barzegari, A., Amouzad Mahdirejei, H., Hanani, M., Esmaeili, M.H., & Salari, A.A. (2023). Adolescent swimming exercise following maternal valproic acid treatment improves cognition and reduces stress-related symptoms in offspring mice: Role of sex and brain cytokines. Physiology & Behavior, 269, 114264. https://doi.org/10.1016/j.phys....
9.
Camuso, S., La Rosa, P., Fiorenza, MT., & Canterini, S. (2022). Pleiotropic effects of BDNF on the cerebellum and hippocampus: Implications for neurodevelopmental disorders. Neurobiology of Disease, 163, 105606. https://doi.org/10.1016/j.nbd.....
10.
Carlezon, W.A.Jr, Kim, W., Missig, G., Finger, B.C., Landino, S.M., Alexander, A.J., Mokler, E.L., Robbins, J.O., Li, Y., Bolshakov, V.Y., McDougle, C.J., & Kim, K.S. (2019). Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Scientific Reports, 9, 16928. https://doi.org/10.1038/s41598....
11.
Chen, Y.S., Zhang, S.M., Yue, C.X., Xiang, P., Li, J.Q., Wei, Z., Xu, L., & Zeng, Y. (2022). Early environmental enrichment for autism spectrum disorder Fmr1 mice models has positive behavioral and molecular effects. Experimental Neurology, 352, 114033. https://doi.org/10.1016/j.expn....
12.
Coley, A.A., & Gao, W.J. (2018). PSD95: A synaptic protein implicated in schizophrenia or autism? Prog Neuropsychopharmacol Biological Psychiatry, 82, 187-194. https://doi.org/10.1016/j.pnpb....
13.
Cooper, R.A., & Simons, J.S. (2019). Exploring the neurocognitive basis of episodic recollection in autism. Psychonomic Bulletin & Review, 26, 163-181. https://doi.org/10.3758/s13423....
14.
Cum, M., Santiago Pérez, J.A., Wangia, E., Lopez, N., Wright, E.S., Iwata, R.L., Li, A., Chambers, A.R., & Padilla-Coreano, N. (2024). A systematic review and meta-analysis of how social memory is studied. Scientific Reports, 14, 2221. https://doi.org/10.1038/s41598....
15.
Desaunay, P., Guillery, B., Moussaoui, E., Eustache, F., Bowler, D.M., & Guénolé, F. (2023). Brain correlates of declarative memory atypicality in autism: a systematic review of functional neuroimaging findings. Molecular Autism, 14, 2. https://doi.org/10.1186/s13229....
16.
Dionísio, A., Espírito, A., Pereira, A.C., Mouga, S., d’Almeida, O.C., Oliveira, G., & Castelo-Branco, M. (2024). Neurochemical differences in core regions of the autistic brain: a multivoxel 1H-MRS study in children. Scientific Reports,14, 2374. https://doi.org/10.1038/s41598....
17.
Duarte-Campos, J.F., Vázquez-Moreno, C.N., Martínez-Marcial, M., Chavarría, A., Ramírez-Carreto, R.J., Velasco Velázquez, M.A., De La Fuente-Granada, M., & González-Arenas, A. (2024).Changes in neuroinflammatory markers and microglial density in the hippocampus and prefrontal cortex of the C58/J mouse model of autism. European Journal of Neuroscience, 59(1), 154-173. https://doi.org/10.1111/ejn.16....
18.
Eissa, N., Azimullah, S, Jayaprakash P, Jayaraj RL, Reiner D, Ojha SK, Beiram R, Stark H, Łażewska D, Kieć-Kononowicz K, & Sadek B. (2019). The dual-active histamine H3 receptor antagonist and acetylcholine esterase inhibitor E100 ameliorates stereotyped repetitive behavior and neuroinflammation in sodium valproate-induced autism in mice. Chemico-Biological Interactions, 312, 108775. https://doi.org/10.1016/j.cbi.....
19.
Elhamid, S.A.A., Alkherkhisy, M.M. & Kasem, R.E. (2024). Assessment of brain-derived neurotrophic factor levels in serum of children with autism spectrum disorders. Middle East Current Psychiatry, 31, 18. https://doi.org/10.1186/s43045....
20.
Fetit, R., Hillary, R.F., Price, D.J., & Lawrie, S.M. (2021). The neuropathology of autism: A systematic review of post-mortem studies of autism and related disorders. Neuroscience & Biobehavioral Reviews, 129, 35-62. https://doi.org/10.1016/j.neub....
21.
Fuchs, C., Gennaccaro, L., Trazzi, S., Bastianini, S., Bettini, S., Lo Martire, V., Ren, E., Medici, G., Zoccoli, G., & Rimondini, R., Ciani, E. (2018). Heterozygous CDKL5 Knockout Female Mice Are a Valuable Animal Model for CDKL5 Disorder. Neural Plasticity, 9726950. https://doi.org/10.1155/2018/9....
22.
Fuentealba, C.R., Fiedler, J.L., Peralta, F.A., Avalos, A.M., Aguayo, F.I., Morgado-Gallardo, K.P., & Aliaga, E.E. (2019). Region-Specific Reduction of BDNF Protein and Transcripts in the Hippocampus of Juvenile Rats Prenatally Treated with Sodium Valproate. Frontiers in Molecular Neuroscience, 12, 261. https://doi.org/10.3389/fnmol....
23.
Gage, F.H. (2019). Adult neurogenesis in mammals. Science, 364(6443), 827-828. https://doi.org/10.1126/scienc....
24.
Georgiou, N., & Spanoudis, G. (2021). Developmental Language Disorder and Autism: Commonalities and Differences on Language. Brain Sciences, 11(5), 589. https://doi.org/10.3390/brains....
25.
Girolamo, T., Shen, L., Monroe Gulick, A., Rice, M.L., & Eigsti, I.M. (2024). Studies assessing domains about structural language in autism vary in reporting practices and approaches to assessment: A systematic review. Autism, 28(7), 1602-1621. https://doi.org/10.1177/136236....
26.
Graham, S. F., Chevallier, O. P., Kumar, P., Trkolu, O. & Bahado-Singh, R. O. (2016). High-resolution metabolomic analysis of ASD human brain uncovers novel biomarkers of disease. Metabolomics, 12, 1-10.https://doi.org/10.1007/s11306....
27.
Griffin, J.W., Bauer, R., & Scherf, K.S. (2021). A quantitative meta-analysis of face recognition deficits in autism: 40 years of research. Psychological Bulletin, 147(3), 268-292. https://doi.org/10.1037/bul000....
28.
Gifford, J.J., Deshpande, P., Mehta, P., Wagner, G.C., & Kusnecov, A.W. (2022). The Effect of Valproic Acid Exposure throughout Development on Microglia Number in the Prefrontal Cortex, Hippocampus and Cerebellum. Neuroscience, 481, 166-177. https://doi.org/10.1016/j.neur....
29.
Hanamsagar, R., Alter, M.D., Block, C.S., Sullivan, H., Bolton, J.L., & Bilbo, S.D. (2017). Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia, 65(9), 1504-1520. https://doi.org/10.1002/glia.2....
30.
Hao, S., Tang, B., Wu, Z., Ure, K., Sun, Y., Tao, H., Gao, Y., Patel, A.J., Curry, D.J., Samaco, R.C., Zoghbi, H.Y., & Tang, J. (2015). Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature, 526, 430-4. https://doi.org/10.1038/nature....
31.
Hashimoto, T., Yokota, S., Matsuzaki, Y., & Kawashima, R. (2021). Intrinsic hippocampal functional connectivity underlying rigid memory in children and adolescents with autism spectrum disorder: A case-control study. Autism, 25(7), 1901-1912. https://doi.org/10.1177/136236....
32.
Hitti, F.L., & Siegelbaum, S.A. (2014). The hippocampal CA2 region is essential for social memory. Nature, 508, 88-92. https://doi.org/10.1038/nature....
33.
Ilchibaeva, T., Tsybko, A., Lipnitskaya, M., Eremin, D., Milutinovich, K., Naumenko, V., & Popova, N. (2023). Brain-Derived Neurotrophic Factor (BDNF) in Mechanisms of Autistic-like Behavior in BTBR Mice: Crosstalk with the Dopaminergic Brain System. Biomedicines, 11(5), 1482. https://doi.org/10.3390/biomed....
34.
Jasien, J.M., Daimon, C.M., Wang, R., Shapiro, B.K., Martin, B., & Maudsley, S. (2014). The effects of aging on the BTBR mouse model of autism spectrum disorder. Frontiers in Aging Neuroscience, 6, 225. https://doi.org/10.3389/fnagi.....
35.
Jeon, S.J., Kwon, H., Bae, H.J., Gonzales, E.L., Kim, J., Chung, H.J., Kim, D.H., Ryu, J.H., & Shin, C.Y. (2022). Agmatine relieves behavioral impairments in Fragile X mice model. Neuropharmacology, 219, 109234. https://doi.org/10.1016/j.neur....
36.
Kurochkin, I., Khrameeva, E., Tkachev, A., Stepanova, V., Vanyushkina, A., Stekolshchikova, E., Li, Q., Zubkov, D., Shichkova, P., Halene, T., Willmitzer, L., Giavalisco, P., Akbarian, S., & Khaitovich, P. (2019). Metabolome signature of autism in the human prefrontal cortex. Communications Biology, 2, 234. https://doi.org/10.1038/s42003....
37.
Leisman, G., Melillo, R., & Melillo, T. (2023). Prefrontal functional connectivities in autism spectrum disorders: A connectopathic disorder affecting movement, interoception, and cognition. Brain Research Bulletin, 198, 65-76. https://doi.org/10.1016/j.brai....
38.
Li, G., Chen, M.H., Li, G., Wu, D., Lian, C., Sun, Q., Rushmore, R.J., % Wang, L. (2023). Volumetric Analysis of Amygdala and Hippocampal Subfields for Infants with Autism. Journal of Autism and Developmental Disorders, 53(6), 2475-2489. https://doi.org/10.1007/s10803....
39.
Li, Y., Shen, M., Stockton, M.E., & Zhao, X. (2019). Hippocampal deficits in neurodevelopmental disorders. Neurobiology of Learning and Memory, 165, 106945. https://doi.org/10.1016/j.nlm.....
40.
Li, Z., Zhu, Y.X., Gu, LJ, & Cheng Y. (2021). Understanding autism spectrum disorders with animal models: applications, insights, and perspectives. Zoological Research, 42(6), 800-824. https://doi.org/10.24272/j.iss....
41.
Libero, L.E., Reid, M.A., White, D.M., Salibi, N., Lahti, A.C., & Kana, R.K. (2016). Biochemistry of the cingulate cortex in autism: An MR spectroscopy study. Autism Research, 9(6), 643-57. https://doi.org/10.1002/aur.15....
42.
Liu, S.H., Shi, X.J., Fan, F.C., & Cheng, Y. (2021). Peripheral blood neurotrophic factor levels in children with autism spectrum disorder: a meta-analysis. Scientific Reports, 11, 15. https://doi.org/10.1038/s41598....
43.
Long, J., Li, H., Liu, Y., Liao, X., Tang, Z., Han, K., Chen, J., & Zhang, H. (2024). Insights into the structure and function of the hippocampus: implications for the pathophysiology and treatment of autism spectrum disorder. Frontiers in Psychiatry, 23,15, 1364858. https://doi.org/10.3389/fpsyt.....
44.
Meng, W. D., Sun, S. J., Yang, J., Chu, R. X., Tu, W., & Liu, Q. (2016). Elevated serum brainderived neurotrophic factor (BDNF) but not BDNF gene Val66Met polymorphism is associated with autism spectrum disorders. Molecular Neurobiology, 54, 1167-1172. https://doi.org/10.1007/s12035....
45.
Pagni, B.A., Walsh, M.J.M., Ofori, E., Chen, K., Sullivan, G., Alvar, J., Monahan, L., Guerithault, N., Delaney, S., & Braden, B.B. (2022). Effects of age on the hippocampus and verbal memory in adults with autism spectrum disorder: Longitudinal versus cross-sectional findings. Autism Research, 10, 1810-1823. https://doi.org/10.1002/aur.27....
46.
Raam, T., McAvoy, K.M., Besnard, A., Veenema, A.H., & Sahay, A. (2018). Author Correction: Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nature Communications, 9(1), 552. https://doi.org/10.1038/s41467....
47.
Rexrode, L.E., Hartley, J., Showmaker, K.C., Challagundla, L., Vandewege, M.W., Martin, B.E., Blair, E., Bollavarapu, R., Antonyraj, R.B., Hilton, K., Gardiner, A., Valeri, J., Gisabella, B., Garrett, M.R., Theoharides, T.C., & Pantazopoulos, H. (2024). Molecular profiling of the hippocampus of children with autism spectrum disorder. Molecular Psychiatry, 29, 1968–1979. https://doi.org/10.1038/s41380....
48.
Sato, M., Nakai, N., Fujima, S., Choe, K.Y., & Takumi, T. (2023). Social circuits and their dysfunction in autism spectrum disorder. Molecular Psychiatry, 28, 3194–3206. https://doi.org/10.1038/s41380....
49.
Segura, M., Pedreno, C., Obiols, J., Taurines, R., Pamias, M., Grunblatt, E., & Gella, A. (2015). Neurotrophin blood-based gene expression and social cognition analysis in patients with autism spectrum disorder. Neurogenetics, 16, 123–131. https://doi.org/10.1007/s10048....
50.
Solmi, M., Song, M., Yon, D.K., Lee, S.W., Fombonne, E., Kim, M.S., Park, S., Lee, M.H., Hwang, J., Keller, R., Koyanagi, A., Jacob, L., Dragioti, E., Smith, L., Correll, C.U., Fusar-Poli, P., Croatto, G., Carvalho, A.F., Oh, J.W., Lee, S., Gosling, C.J., Cheon, K.A., Mavridis, D., Chu, C.S., Liang, C.S., Radua, J., Boyer, L., Fond, G., Shin, J.I., & Cortese, S. (2022). Incidence, prevalence, and global burden of autism spectrum disorder from 1990 to 2019 across 204 countries. Molecular Psychiatry, 27, 4172-4180. https://doi.org/10.1038/s41380....
51.
Solomon, M., Ragland, J.D., Niendam, T.A., Lesh, T.A., Beck, J.S., Matter, J.C., Frank, M.J., & Carter, C.S. (2015). Atypical Learning in Autism Spectrum Disorders: A Functional Magnetic Resonance Imaging Study of Transitive Inference. Journal of the American Academy of Child & Adolescent Psychiatry, 54(11), 947-55. https://doi.org/10.1016/j.jaac....
52.
Staudigl, T., Leszczynski, M., Jacobs, J., Sheth, S.A., Schroeder, C.E., Jensen O., & Doeller, C.F. (2018). Hexadirectional Modulation of High-Frequency Electrophysiological Activity in the Human Anterior Medial Temporal Lobe Maps Visual Space. Current Biology, 28(20), 3325-3329.e4. https://doi.org/10.1016/j.cub.....
53.
Sun, Y., Gao, Y., Tidei, J.J., Shen, M., Hoang, J.T., Wagner, D.F., & Zhao, X. (2019). Loss of MeCP2 in immature neurons leads to impaired network integration. Human Molecular Genetics, 28(2), 245-257. https://doi.org/10.1093/hmg/dd....
54.
Thomson, A.R., Pasanta, D., Arichi, T., & Puts, N.A. (2024). Neurometabolic differences in Autism as assessed with Magnetic Resonance Spectroscopy: A systematic review and metaanalysis. Neuroscience & Biobehavioral Reviews, 62, 105728. https://doi.org/10.1016/j.neub....
55.
Trontel, H.G., Duffield, T.C., Bigler, E.D., Froehlich, A., Prigge, M.B., Nielsen, J.A., Cooperrider, J.R., Cariello, A.N., Travers, B.G., Anderson, J.S., Zielinski, B.A., Alexander, A., Lange, N., & Lainhart, J.E. (2013). Fusiform correlates of facial memory in autism. Behavioral sciences (Basel, Switzerland), 3(3), 348-71. https://doi.org/10.3390/bs3030....
56.
Tsilioni, I., Patel, A.B., Pantazopoulos, H., Berretta, S., Conti, P., Leeman, S.E., & Theoharides, T.C. (2019). IL-37 is increased in the brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin. Proceedings of the National Academy of Sciences (PNAS), 116(43), 21659-21665. https://doi.org/10.1073/pnas.1....
57.
Wan, L., Yang, G., & Yan, Z. (2024). Identification of a molecular network regulated by multiple ASD high-risk genes. Human Molecular Genetics, 33(13), 1176-1185. https://doi.org/10.1093/hmg/dd....
58.
Wang, Y., Zhang, Y.B., Liu, L.L., Cui, J.F., Wang, J., Shum, D.H, van Amelsvoort, T., & Chan, R.C. (2017). A Meta-Analysis of Working Memory Impairments in Autism Spectrum Disorders. Neuropsychology Review, 27, 46-61. https://doi.org/10.1007/s11065....
59.
Zhao, H., Mao, X., Zhu, C., Zou, X., Peng, F., Yang, W., Li, B., Li, G., Ge, T., & Cui, R. (2022). GABAergic System Dysfunction in Autism Spectrum Disorders. Frontiers in Cell and Developmental Biology, 9, 781327. https://doi.org/10.3389/fcell.....
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.